-
Butts Research
- Meet the Team
- Metabolomic Pathways of Symptoms in Black Adults with Heart Failure
- Skeletal Muscle in Heart Failure with Preserved Ejection Fraction
- Inflammasome in Heart Failure
- Exercise and Heart Failure
- Telomeres in Heart Failure
- Heart Failure Research Studies
- Publications
- Presentation Abstracts
- Atlanta Heart Failure Knowledge Test
- PREVAIL Lab
- MVP Study
- CARDI Study
- Past Studies
- Pathophysiology Videos
- Genetics Workshop (UNC)
- Omics Workshop (SNRS 2023)
- Diabetes Prevention and Insulin
- AHFKT
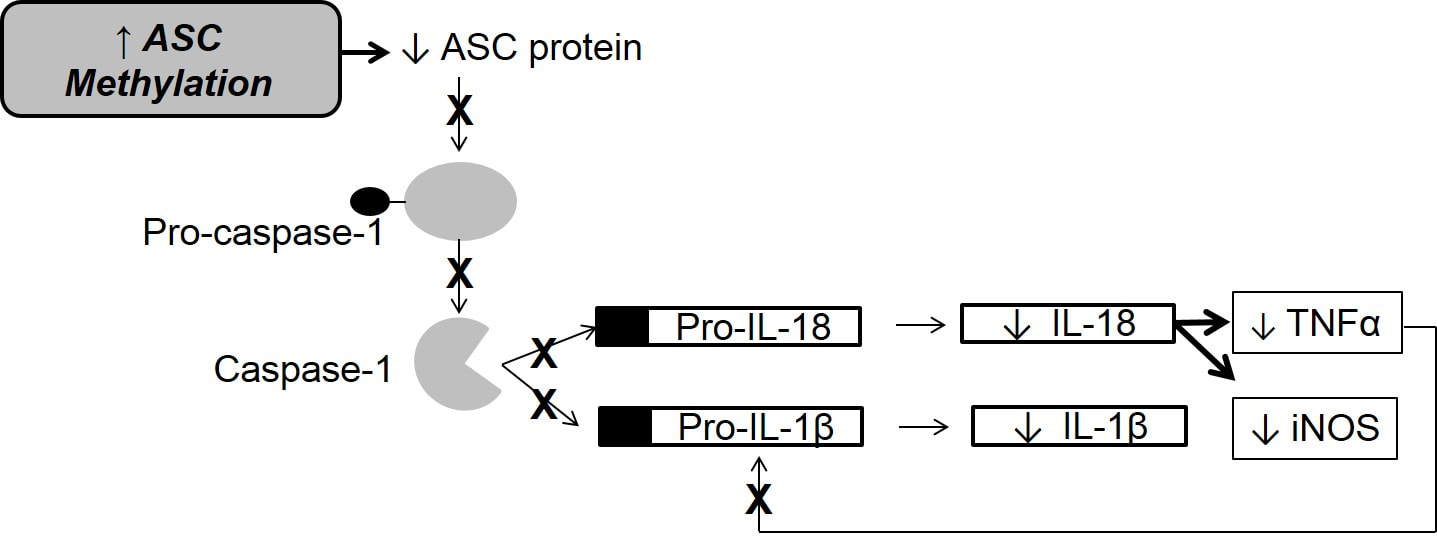
Patients with heart failure continue to suffer adverse health consequences despite advances in therapies over the last two decades. Identification of novel therapeutic targets that may attenuate disease progression is therefore needed. The inflammasome may play a central role in modulating chronic inflammation and in turn affecting heart failure progression. The inflammasome is a complex of intracellular interaction proteins that trigger maturation of pro-inflammatory cytokines interleukin-1beta and interleukin-18 to initiate the inflammatory response. This response is amplified through production of tumor necrosis factor α and activation of inducible nitric oxide synthase.
Heart failure (HF) results from structural or functional impairment of ventricular function leading to inability to meet the body needs for adequate blood supply without raising the filling ventricular pressures over normal limits. Heart failure remains a leading cause of morbidity and mortality in the United States, with approximately 50% 5-year mortality risk, and is the leading cause of hospitalization among individuals over age 65. Thus, identification of novel pathophysiological pathways and potential therapeutic targets that may slow disease progression are urgently needed.
Heart failure is associated with a chronic, low-grade, sterile inflammation characterized by the formation and activation of a protein complex, the inflammasome, which activates inflammatory cytokines that promote cardiac hypertrophy and myocardial apoptosis. Heart failure progression results from cytokines worsening hemodynamic abnormalities or by exerting toxic effects directly on the cardiac muscle. Increased levels of inflammatory cytokines are associated with increased HF progression, severity and death.
IL-1β and IL-18 are pleiotropic, inflammatory cytokines theorized to play a prognostic and mechanistic role in HF; increased levels have been shown to significantly contribute to worsening HF severity and mortality. IL-1β and IL-18 increase with acute HF decompensation, suggesting a role in myocardial dysfunction. IL-18 induces the production of tumor necrosis factor-alpha (TNFα). IL-1β is expressed as proform 1L-1β upon immune activation, while IL-18 is constitutively expressed as proform IL-18; both molecules require caspase-1 dependent proteolytic cleavage for activation. Caspase-1 is recruited by an adaptor molecule, ASC, which leads to IL-1β and IL-18 activation. ASC expression is epigenetically controlled by CpG methylation. Increased methylation inversely correlates with ASC expression.
For more information see: Butts B, Gary RA, Dunbar SB, Butler J. The Importance of NLRP3 Inflammasome in Heart Failure. J Card Fail. 2015;21(7):586-593.
Heart failure is associated with a chronic, low-grade, sterile inflammation characterized by the formation and activation of a protein complex, the inflammasome, which activates inflammatory cytokines that promote cardiac hypertrophy and myocardial apoptosis. Heart failure progression results from cytokines worsening hemodynamic abnormalities or by exerting toxic effects directly on the cardiac muscle. Increased levels of inflammatory cytokines are associated with increased HF progression, severity and death.
IL-1β and IL-18 are pleiotropic, inflammatory cytokines theorized to play a prognostic and mechanistic role in HF; increased levels have been shown to significantly contribute to worsening HF severity and mortality. IL-1β and IL-18 increase with acute HF decompensation, suggesting a role in myocardial dysfunction. IL-18 induces the production of tumor necrosis factor-alpha (TNFα). IL-1β is expressed as proform 1L-1β upon immune activation, while IL-18 is constitutively expressed as proform IL-18; both molecules require caspase-1 dependent proteolytic cleavage for activation. Caspase-1 is recruited by an adaptor molecule, ASC, which leads to IL-1β and IL-18 activation. ASC expression is epigenetically controlled by CpG methylation. Increased methylation inversely correlates with ASC expression.
For more information see: Butts B, Gary RA, Dunbar SB, Butler J. The Importance of NLRP3 Inflammasome in Heart Failure. J Card Fail. 2015;21(7):586-593.
Epigenetic Control of Inflammatory Pathways in Heart Failure
Study Aims:
This study examined epigenetic control of ASC (apoptosis-associated spec-like protein containing a caspase recruitment domain) methylation and whether this initiates a downstream change in pro-inflammatory cytokines (interleukin-1β [IL-1β], interleukin-18 [IL-18], tumor necrosis alpha [TNF-α]) known to worsen outcomes in persons with heart failure (HF).
This study examined inherent mechanisms of inflammasome modulation, epigenetic control of a key inflammasome protein, ASC, in patients with HF. This study was performed on biospecimens stored from a HF cohort study conducted at Emory University (TACC – The Atlanta Cardiomyopathy Consortium) that enrolled 330 patients with a mean follow-up of over 3 years and a substantial number of outcomes events (mortality, transplantation, left ventricular assist device placement, and hospitalization.
Aim 1: Determine the correlation between methylation of apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and inflammatory cytokines, IL-1β, IL-18 and TNF-α, in persons with HF.
Hypothesis 1a: Levels of ASC methylation will inversely correlate with plasma IL-1β and IL-18 in HF.
Hypothesis 1b: Levels of ASC methylation will be inversely correlated with plasma TNFα in HF.
Aim 2: Examine the differences in methylation of ASC among patients with HF and reduced ejection fraction (HFrEF) and those with HF and preserved ejection fraction (HFpEF).
Research Question : Do patients with HFrEF and those with HFpEF have different levels of ASC methylation?
Aim 3: Examine the relationships between patient-reported outcomes and proposed inflammatory pathway.
Research Question 3: Do levels of ASC methylation, IL-1β, IL-18 and TNFα correlate with patient-reported outcomes?
Aim 4: Determine the relationships between functional capacity and ASC methylation and inflammatory cytokines in persons with heart failure.
Hypothesis 4a: Levels of ASC methylation will be positively correlated with 6-minute walk test in patients with HF.
Hypothesis 4b: Plasma IL-1β, IL-18 and TNFα will be inversely correlated with 6-minute walk test in patients with HF.
Results:
Background: Heart failure (HF) is associated with inflammation characterized by the formation the inflammasome, which triggers maturation of inflammatory cytokines. ASC, a vital component of the inflammasome, is controlled through epigenetic modification, which may be a candidate pathway for worsening HF. This study examined the inflammasome pathway in HF and the relationships between ASC CpG methylation and outcomes in HF.
Methods and Results: Stored samples from 155 HF outpatients (ejection fraction 29.9±14.9) were analyzed for % methylation of seven CpG sites in the intron region preceding exon-1 of the ASC gene. ASC methylation was inversely related to ASC mRNA (r=-.33,P<.001) and protein (r=-.464,P<.001). ASC methylation had a positive linear relationship with ejection fraction (r=.85,P<.001), quality of life (r=.83,P<.001), and six-minute walk test (r=.59,P=.023), and a negative linear relationship with depression (r=-.81,P<.001) and anxiety (r=-.75,P<.001). Higher ASC methylation was associated with a lower risk for clinical events (HR 0.16,P=.025), while higher protein (HR=1.78,P=.045) and mRNA expression (HR=1.18,P=.05) were associated with a greater risk.
Conclusion: Increased methylation of CpG sites in the intron region of ASC is associated with improved outcomes in HF. The associated decrease in ASC expression implicates this inflammatory mediator as a possible driver of HF outcomes and may represent a therapeutic target.
Publication: Butts B, Gary RA, Dunbar SB, Butler J. The Importance of NLRP3 Inflammasome in Heart Failure. J Card Fail. 2015;21(7):586-593.
Presentations:
American Heart Association Scientific Sessions, November 2015, Orlando, FL
Heart Failure Society of America, September 2015, National Harbor, MD
Study Aims:
This study examined epigenetic control of ASC (apoptosis-associated spec-like protein containing a caspase recruitment domain) methylation and whether this initiates a downstream change in pro-inflammatory cytokines (interleukin-1β [IL-1β], interleukin-18 [IL-18], tumor necrosis alpha [TNF-α]) known to worsen outcomes in persons with heart failure (HF).
This study examined inherent mechanisms of inflammasome modulation, epigenetic control of a key inflammasome protein, ASC, in patients with HF. This study was performed on biospecimens stored from a HF cohort study conducted at Emory University (TACC – The Atlanta Cardiomyopathy Consortium) that enrolled 330 patients with a mean follow-up of over 3 years and a substantial number of outcomes events (mortality, transplantation, left ventricular assist device placement, and hospitalization.
Aim 1: Determine the correlation between methylation of apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and inflammatory cytokines, IL-1β, IL-18 and TNF-α, in persons with HF.
Hypothesis 1a: Levels of ASC methylation will inversely correlate with plasma IL-1β and IL-18 in HF.
Hypothesis 1b: Levels of ASC methylation will be inversely correlated with plasma TNFα in HF.
Aim 2: Examine the differences in methylation of ASC among patients with HF and reduced ejection fraction (HFrEF) and those with HF and preserved ejection fraction (HFpEF).
Research Question : Do patients with HFrEF and those with HFpEF have different levels of ASC methylation?
Aim 3: Examine the relationships between patient-reported outcomes and proposed inflammatory pathway.
Research Question 3: Do levels of ASC methylation, IL-1β, IL-18 and TNFα correlate with patient-reported outcomes?
Aim 4: Determine the relationships between functional capacity and ASC methylation and inflammatory cytokines in persons with heart failure.
Hypothesis 4a: Levels of ASC methylation will be positively correlated with 6-minute walk test in patients with HF.
Hypothesis 4b: Plasma IL-1β, IL-18 and TNFα will be inversely correlated with 6-minute walk test in patients with HF.
Results:
Background: Heart failure (HF) is associated with inflammation characterized by the formation the inflammasome, which triggers maturation of inflammatory cytokines. ASC, a vital component of the inflammasome, is controlled through epigenetic modification, which may be a candidate pathway for worsening HF. This study examined the inflammasome pathway in HF and the relationships between ASC CpG methylation and outcomes in HF.
Methods and Results: Stored samples from 155 HF outpatients (ejection fraction 29.9±14.9) were analyzed for % methylation of seven CpG sites in the intron region preceding exon-1 of the ASC gene. ASC methylation was inversely related to ASC mRNA (r=-.33,P<.001) and protein (r=-.464,P<.001). ASC methylation had a positive linear relationship with ejection fraction (r=.85,P<.001), quality of life (r=.83,P<.001), and six-minute walk test (r=.59,P=.023), and a negative linear relationship with depression (r=-.81,P<.001) and anxiety (r=-.75,P<.001). Higher ASC methylation was associated with a lower risk for clinical events (HR 0.16,P=.025), while higher protein (HR=1.78,P=.045) and mRNA expression (HR=1.18,P=.05) were associated with a greater risk.
Conclusion: Increased methylation of CpG sites in the intron region of ASC is associated with improved outcomes in HF. The associated decrease in ASC expression implicates this inflammatory mediator as a possible driver of HF outcomes and may represent a therapeutic target.
Publication: Butts B, Gary RA, Dunbar SB, Butler J. The Importance of NLRP3 Inflammasome in Heart Failure. J Card Fail. 2015;21(7):586-593.
Presentations:
American Heart Association Scientific Sessions, November 2015, Orlando, FL
Heart Failure Society of America, September 2015, National Harbor, MD
ASC Methylation and Aerobic Capacity in Heart Failure
Background: Aerobic capacity, as measured by peak oxygen uptake (V̇O2), is one of the most powerful predictors of prognosis in heart failure (HF). Inflammation is a key factor contributing to alterations in aerobic capacity, and interleukin (IL)-1 cytokines are implicated in this process. The adaptor protein ASC is necessary for inflammasome activation of IL-1β and IL-18. ASC expression is controlled through epigenetic modification; lower ASC methylation is associated with worse outcomes in HF. The purpose of this study is to examine the relationships between ASC methylation, IL-1β, and IL-18 with peak V̇O2 in persons with HF.
Methods: This study examined the relationship between ASC methylation, IL-1β, and IL-18 with peak V̇O2 in 54 stable outpatients with HF. All participants were NYHA class II or III, not engaged in an exercise program, and physically able to complete an exercise treadmill test.
Results: Mean peak V̇O2 was 16.68 ± 4.7 ml/kg/min. Peak V̇O2 was positively associated with mean percent ASC methylation (r=.47, p=.001) and negatively associated with IL-1β (r=-.38, p=.007). Multiple linear regression models demonstrated that peak V̇O2 increased by 2.30 ml/kg/min for every 1% increase in ASC methylation and decreased by 1.91 ml/kg/min for every 1 pg/mL increase in plasma IL-1β.
Conclusions: Mean percent ASC methylation and plasma IL-1β levels are associated with clinically meaningful differences in peak V̇O2 in persons with HF. Inflammasome activation may play a mechanistic role in determining aerobic capacity. ASC methylation is a potentially modifiable mechanism for reducing the inflammatory response, thereby improving aerobic capacity in HF.
Publication: Butts B, Butler J, Dunbar SB, Corwin EJ, Gary RA. ASC Methylation and Interleukin-1beta Are Associated with Aerobic Capacity in Heart Failure. Med Sci Sports Exerc. 2017;49(6):1072-1078.
Presented at: American Heart Association Scientific Sessions, November 2016, New Orleans, LA
Background: Aerobic capacity, as measured by peak oxygen uptake (V̇O2), is one of the most powerful predictors of prognosis in heart failure (HF). Inflammation is a key factor contributing to alterations in aerobic capacity, and interleukin (IL)-1 cytokines are implicated in this process. The adaptor protein ASC is necessary for inflammasome activation of IL-1β and IL-18. ASC expression is controlled through epigenetic modification; lower ASC methylation is associated with worse outcomes in HF. The purpose of this study is to examine the relationships between ASC methylation, IL-1β, and IL-18 with peak V̇O2 in persons with HF.
Methods: This study examined the relationship between ASC methylation, IL-1β, and IL-18 with peak V̇O2 in 54 stable outpatients with HF. All participants were NYHA class II or III, not engaged in an exercise program, and physically able to complete an exercise treadmill test.
Results: Mean peak V̇O2 was 16.68 ± 4.7 ml/kg/min. Peak V̇O2 was positively associated with mean percent ASC methylation (r=.47, p=.001) and negatively associated with IL-1β (r=-.38, p=.007). Multiple linear regression models demonstrated that peak V̇O2 increased by 2.30 ml/kg/min for every 1% increase in ASC methylation and decreased by 1.91 ml/kg/min for every 1 pg/mL increase in plasma IL-1β.
Conclusions: Mean percent ASC methylation and plasma IL-1β levels are associated with clinically meaningful differences in peak V̇O2 in persons with HF. Inflammasome activation may play a mechanistic role in determining aerobic capacity. ASC methylation is a potentially modifiable mechanism for reducing the inflammatory response, thereby improving aerobic capacity in HF.
Publication: Butts B, Butler J, Dunbar SB, Corwin EJ, Gary RA. ASC Methylation and Interleukin-1beta Are Associated with Aerobic Capacity in Heart Failure. Med Sci Sports Exerc. 2017;49(6):1072-1078.
Presented at: American Heart Association Scientific Sessions, November 2016, New Orleans, LA
Effects of Exercise on ASC Methylation and IL-1 Cytokines in Heart Failure.
Introduction: Inflammation contributes to heart failure (HF) progression and the interleukin (IL)-1 cytokine IL-1β is implicated in this process. The adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) is necessary for inflammasome activation of IL-1β. Lower ASC methylation is associated with worse outcomes in HF. The purpose of this study was to examine the effects of exercise on changes in ASC methylation and activation of the IL-1 family cytokine IL-1β in persons with HF.
Methods: Participants (N = 54) were randomized to receive exercise intervention (n = 38) or attention control (n = 16) for 3 months. Percent methylation of the ASC gene, plasma IL-1β, and ASC mRNA and were obtained at baseline, 3 months, and 6 months.
Results: ASC methylation was higher in the exercise group as compared to control at 3 months (6.10% ± 0.5% vs 5.80% ± 0.4%; P = 0.04) and 6 months (6.07 ± 0.4 vs 5.82 ± 0.4; P = 0.04). Plasma IL-1β was lower in the exercise group at 3 months (1.43 ± 0.5 pg·mL vs 2.09 ± 1.3 pg·mL; P = 0.02) and 6 months (1.49 ± 0.5 pg·mL vs 2.13 ± 1.4 pg·mL; P = 0.004). ASC mRNA expression was negatively associated with ASC methylation at baseline (r = -0.97, P = 0.001), 3 months (r = -0.90, P = 0.001), and 6 months (r = -0.81, P = 0.001). ASC mRNA was lower than baseline at 3 months (P = 0.004) and 6 months (P = 0.002) among those in the exercise group. ASC methylation was positively associated with 6-min walk test at baseline (r = 0.517, P < 0.001), 3 months (r = 0.464, P = 0.004), and 6 months (r = 497, P = 0.05).
Conclusions: Exercise was related to increased mean percent ASC methylation and decreased IL-1β and ASC mRNA gene expression in HF. Epigenetic regulation of ASC can be a biological mechanism by which exercise can promote better outcomes in HF.
Publication: Butts B, Butler J, Dunbar SB, Corwin E, Gary RA. Effects of Exercise on ASC Methylation and IL-1 Cytokines in Heart Failure. Med Sci Sports Exerc. 2018;50(9):1757-1766.
Introduction: Inflammation contributes to heart failure (HF) progression and the interleukin (IL)-1 cytokine IL-1β is implicated in this process. The adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) is necessary for inflammasome activation of IL-1β. Lower ASC methylation is associated with worse outcomes in HF. The purpose of this study was to examine the effects of exercise on changes in ASC methylation and activation of the IL-1 family cytokine IL-1β in persons with HF.
Methods: Participants (N = 54) were randomized to receive exercise intervention (n = 38) or attention control (n = 16) for 3 months. Percent methylation of the ASC gene, plasma IL-1β, and ASC mRNA and were obtained at baseline, 3 months, and 6 months.
Results: ASC methylation was higher in the exercise group as compared to control at 3 months (6.10% ± 0.5% vs 5.80% ± 0.4%; P = 0.04) and 6 months (6.07 ± 0.4 vs 5.82 ± 0.4; P = 0.04). Plasma IL-1β was lower in the exercise group at 3 months (1.43 ± 0.5 pg·mL vs 2.09 ± 1.3 pg·mL; P = 0.02) and 6 months (1.49 ± 0.5 pg·mL vs 2.13 ± 1.4 pg·mL; P = 0.004). ASC mRNA expression was negatively associated with ASC methylation at baseline (r = -0.97, P = 0.001), 3 months (r = -0.90, P = 0.001), and 6 months (r = -0.81, P = 0.001). ASC mRNA was lower than baseline at 3 months (P = 0.004) and 6 months (P = 0.002) among those in the exercise group. ASC methylation was positively associated with 6-min walk test at baseline (r = 0.517, P < 0.001), 3 months (r = 0.464, P = 0.004), and 6 months (r = 497, P = 0.05).
Conclusions: Exercise was related to increased mean percent ASC methylation and decreased IL-1β and ASC mRNA gene expression in HF. Epigenetic regulation of ASC can be a biological mechanism by which exercise can promote better outcomes in HF.
Publication: Butts B, Butler J, Dunbar SB, Corwin E, Gary RA. Effects of Exercise on ASC Methylation and IL-1 Cytokines in Heart Failure. Med Sci Sports Exerc. 2018;50(9):1757-1766.
|
Funding:
NIH NINR F31NR015180 Heart Failure Society of America NIH NINR T32NR012715 (PI - Sandra Dunbar, 09/2012 - 08/2014) |
Proudly powered by Weebly